USES OF PERIODIC TABLE, LAW COLMN AND IMPORTANT METAL AND NON METAL

USES OF PERIODIC TABLE, LAW COLMN AND IMPORTANT METAL AND NON METAL
The following are short history about exaggerating of periodic table
In 1817, the German scientist Johann Dobereiner noticed that calcium, strontium and barium had similar properties, and that the atomic weight of strontium was halfway between the other two. He found the same pattern with chlorine, bromine and iodine and also with lithium, sodium and potassium. So, he put forward the law of Triads: “If elements are arranged in groups of three in order of increasing atomic weights, having similar properties, then the atomic weight of the middle element is the arithmetic mean of the atomic weights of the other two elements”, E.g.
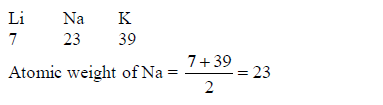
The following are examples of Dobereiner's triads:(Lithium, Sodium and Potassium)(Calcium, Strontium and Barium)(Chlorine, Bromine and Iodine) and(Iron, Cobalt and Nickel)
The Law of Octaves
In 1863 John Newlands, an English chemist noted that there were many pairs of similar elements. In each pair, the atomic weights differed by a multiple of 8. So, he produced a table with the elements in order of increasing atomic weights, and put forward the Law of Octaves: “If elements are arranged in order of their increasing atomic weights, the properties of the 8th element, starting from a given one, are a kind of repetition of the first element”.
This finding was comparable to the 8th note of music, hence the use of the word "octave".
This was the first table to show a periodic or repeating pattern of properties. But it was not widely accepted because there were too many inconsistencies. For example, he put copper and sodium in the same group, even though have very different properties. Also iron was placed in the same group as oxygen and sulphur.
The Periodic Law
Dmitri Mendeleev was born in Siberia, Russia, in 1834. By the time he was 32, he was a professor of Chemistry. In 1869 Mendeleev advanced the work done by Newlands and contributed very useful new ideas. He began by listing all the known elements in order of increasing atomic mass. He spotted that elements with similar properties appear at regular intervals or periods down the list. His findings were the basis for the Periodic Law: “The properties of elements are a periodic function of their atomic masses”.
Mendeleev placed similar elements into groups. He realized that not all elements had been discovered. So he left gaps for new ones in the correct places in his table. He also swapped the order of some elements to make them fit better. He predicted the properties of the missing elements from the properties of the elements above and below them in the table. He also listed separately some elements which did not appear to fit into any group i.e. iron, cobalt, nickel, etc.
Table 6.1: Mendeleev’s short form of the Periodic Table
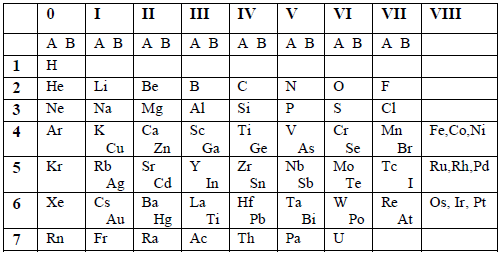
The table had 9 vertical columns which he called Groups. The groups were numbered from 0 to 8. The elements in group 0 were not known by then, but were discovered later on. Groups 1 to 7 were subdivided into A and B subgroups. Group 0 included the transition elements. Noble gases were later placed in group 0.
There were 7 horizontal rows which he called periods. All vacant positions in the table stood for new elements yet to be discovered.
Usefulness of Mendeleev's classification
- The table summarized a large amount of information about the elements based on their chemical properties.
- The table was very useful in predicting the existence and properties of undiscovered elements, for which gaps had been left in the table.
- The table was also used in checking relative atomic masses of elements.
Limitations of Mendeleev's classification
- In three cases, pairs of elements had to be included in one group based on inverse order of their atomic weights so as to fit into groups of elements having similar properties. These pairs were argon (39.9) and potassium (39.1), cobalt (58.9) and nickel (58.9); plus tellurium (127.5) and iodine (126.9). This difficulty was resolved when the basis of classification was based on the atomic number instead of the atomic mass.
- The elements that were placed in group VIII formed an incompatible mixture.
- The placing of two different families in one group e.g. K and Cu; Ca and Zn, etc.
The periodic table is the chemists map. It helps you understand the patterns in chemistry. Today we take it for granted. But it took hundreds of years, and work of hundreds of chemists, to develop.
The Modern Periodic Table is similar to that of Mendeleev, but contains several improvements. Elements are arranged in order of atomic number instead of atomic mass. This means that elements no longer have to swap places to fit correctly. Many new elements have been discovered and slotted into the spaces left by Mendeleev. Also metals and non-metals are clearly separated. The Modern Periodic Table is shown in Figure 6.1.
Figure 6.1: The Modern Periodic Table
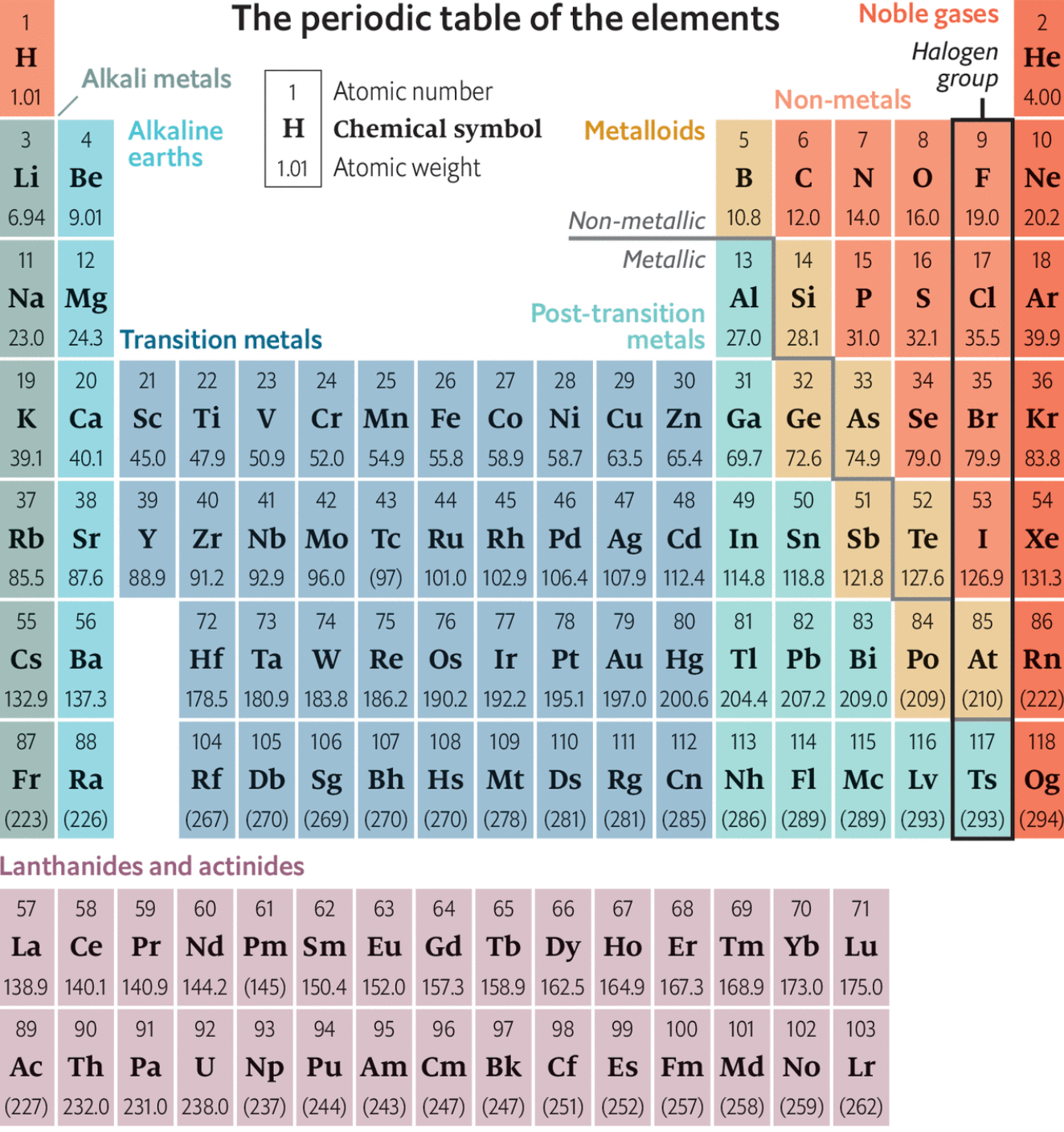
The long form of the periodic table is the commonly used form of the periodic table. The elements in the table are arranged based on their atomic weights, starting from hydrogen (1), helium (2), lithium (3), beryllium (4) and so on. The elements appear in vertical columns and horizontal rows.
The vertical columns in the table are called Groups, numbered I, II, III, IV, V, VI, VII and 0, which is also known as group VIII. Group I contains the elements lithium (L), sodium (Na), rubidium (Rb), caesium (Cs) and francium (Fr). Group II consists of elements starting from beryllium (Be) down to radium (Ra). Some of the groups have special names.
- Group I is often called the alkali metals.
- Group II the alkaline earth metals.
- Group VII the halogens.
- Group 0 the noble gases.
The transition metals (or elements) form a separate block in the middle of the periodic table between group II and III. The atoms of these elements have more complicated electron arrangements. Note that the group contains many common metals such as iron (Fe), Nickel (Ni), copper (Cu), and Zinc (Zn). One of the interesting properties of these elements is that they form coloured compounds.
Main features of the Modern Periodic Table
- The elements in the table are placed in order of their atomic numbers instead of their atomic masses.
- There are a total of 18 groups and 7 periods.
- There are 5 blocks of similar elements in the periodic table as shown in figure 6.2.
- The normal (non-transition) elements (groups 1-7) have their outermost shells incomplete, meaning that they can allow additional electrons to enter into their outermost orbital (valency shell). But each of their inner shells is complete.
- The transition metals have their outermost as well as their penultimate (second last) shells incomplete.
- Elements of group 0 (noble gases) have their shells complete. These elements show little reactivity. That is why they wereonce called „inert‟ gases because they are very unreactive; or „rare gases‟ because they were rarely found.
- Gaps left by Mendeleev for undiscovered elements (now occupied by the transition elements and the noble gases) have been filled by the respective elements following their discovery. Man-made elements have also found a place in the periodic table.
- Metals have been clearly separated from non-metals. Metalloids or semi metals (poor metals) have also been included. Metalloids are elements whose properties are intermediate between metals and non-metals. They include boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb) and tellurium (Te). In some publications, germanium and antimony are usually classed as poor metals and the rest as non-metals.

Periodicity
The Concept of Periodicity
Explain the concept of periodicity
Consider the electronic configuration of the first twenty elements of the periodic table shown in the table below.
Table 6.3: Electronic configurations of the first 20 elements
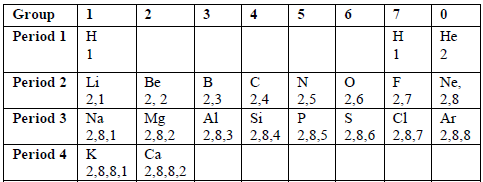
You will notice that elements in the same vertical columns (groups) have the same number of electrons in the outermost shells of their atoms. Because the outer electrons determine the chemical properties of an element, then the elements in each period tend to resemble each other closely in chemical behaviour. For instance, the noble gases, He, Ne and Ar show a chemical inertness which is characterised by the stable outer electron octet or duplet. Due to this reason, the compounds of the noble gases with other elements have not been found.
Attempts to classify elements by arranging them in order of increasing atomic weights shows that the properties of elements were periodic. This means elements with similar or comparable properties appear after a certain specific interval in a given arrangement. The occurrence of successive groups of elements showing strong chemical similarity in this way is called periodicity.
Therefore, periodicity is the repetition of similar chemical properties of elements after a certain specific interval in a given arrangement. The repetition in properties is due to repetition of similar electronic configuration of outermost shells of elements after certain intervals.
Generally periodic table help a scientist in making good preparation of chemical before they react
Comment / Reply From
You May Also Like
Popular Posts
Newsletter
Subscribe to our mailing list to get the new updates!
Categories
- Places and Regions (349)
- Health & Science (3559)
- Jobs (188)
- Work Life (286)
- Opinions (426)
- Real estate & Properties (121)
- Shipping & Logistics (64)
- Sex & Relationships (1755)
- Movies & Animation (6102)
- Comedy (229)
- Travel and Events (427)
- Gaming (1185)
- History and Facts (1296)
- People and Nations (1020)
- Science and Technology (3704)
- Arts & Entertainment (1810)
- Life Style (3627)
- Education (3386)
- Economics and Trade (1950)
- Others (5396)
- News and Politics (3218)
- Cars and Vehicles (430)
- Pets and Animals (326)
- Digital Marketing & Web Develpment (4)
- Robotics, VR & AR (0)
- DFTUntoldStories (1)
- Celebrities (83)
- Mobile Solutions & Apps (0)
- Ecommerce & Clean Tech (0)
- Artificial Inteligence & IoT (0)
- Big Data & Cyber Security (0)
- Business (1780)
- Palscity Show (0)
- Sports Show (0)
- Politics & Leadership Show (0)
- Digitally Fit Show (0)
- Entertainment & Lifestyle Show (0)
- Business Show (1)
- In The Morning Show (0)
- DFT Reels & Shorts (0)
- Natural & Food (1141)
- People and Culture (11)
- Sports (1906)
- Fashion (116)
- Gossip (55)
- Music (116)